
Summer 2012 - Vol.7 - No.2
Lung Cancer Screening with Low-dose CT Imaging
Edgar Fearnow, MD
Section Chief, Computed Tomography,
Lancaster General Hospital
Despite recent declines in the incidence of lung cancer related to the decreasing prevalence of smoking in our society, lung cancer remains the most commonly diagnosed cancer that affects both men and women, and is the leading cause of cancer death in the United States. Given its high prevalence and incidence, and the very low cure rates after surgery, a method to screen for lung cancer has been sought for many years. It was shown quite early that the conventional chest x-ray, although cheap and easily performed, missed most early cancers and generally detected tumors in the later stages of progression. Sputum cytology was likewise found to be ineffective.
It was only with the advent and widespread use of computed tomography (CT) that a tool of sufficient sensitivity became available. In November 2010 the National Lung Cancer Screening Trial (NLST),1 which is described further below, was halted by the NIH when analysis of the data showed a 20% reduction in lung cancer mortality and a 7% reduction in all-cause mortality when screening CT was performed in a selected high risk population. In October 2011 the National Comprehensive Cancer Network (NCCN) published guidelines recommending the use of low-dose CT (LDCT ) for lung cancer screening.2 Understanding the risks and benefits of screening will assist patients and physicians in determining suitability for screening and management of the findings from the CT scans that are performed.
Benefits of Low Dose CT Technique
A significant saving in CT dose is obtained by lowering the tube voltage to 120 - 140 kVp (peak kiloVoltage) and the tube current time product to 40 - 80 mAs (milliAmpere seconds). The resultant exposure is approximately 1.5 mSv, whereas conventional CT results in an exposure of 5 -8 mSv (milliSieverts). Newer iterative reconstruction techniques will result in even lower doses. LDCT scans are reconstructed at 1 – 1.5 mm slice thickness which improves detection and characterization of lesions. At some centers including LGH, computer-aided diagnosis (CAD) software is used to improve nodule detection. LDCT is not, however, currently recommended for assessment of mediastinal or other extrapulmonary abnormalities.
CT Screening Trials
Early CT screening trials were non-randomized but showed encouraging results. Of these studies the International Early Lung Cancer Action Program (I-ELCAP) was the largest, and included 31,567 high risk patients who were assessed with baseline and annual LDCT. Of the lung cancers detected, 85% were stage I with a 92% actuarial 10-year survival for the stage I cancers.
To address concerns from non-randomized studies the NCI launched the National Lung Screening Trial (NLST) in 2002.1 The NLST was a prospective randomized trial that compared annual LDCTs to annual chest x-rays for 3 years. Over 50,000 high risk patients age 55-74 who had at least a 30-pack-year smoking history were included. As noted, the trial was halted in 2010 due to the compelling results which showed a 20% reduction in lung cancer specific mortality and a 7% reduction in all-cause mortality.
27% of individuals in NLST, had an abnormal screening exam. Of these patients, 4% had lung cancer, which means that 96% of the abnormal findings were false-positives. Most of these false positive findings were small nodules, 4 to 8 mm in size, for which serial CT scans were performed to insure long-term stability. In sum, the NLST showed that 320 high-risk individuals needed to be screened to prevent 1 cancer death.
NCCN Lung Cancer Screening Guidelines
Based on the NLST results, in October 2011 the NCCN published the first lung cancer screening guidelines recommending the use of screening CT.2 Screening is recommended (category 1 evidence) for individuals age 55-74 with at least a 30 pack-year smoking history; former smokers must have quit within the past 15 years. Annual screening is recommended for these high risk individuals until they are 74 years old, but in general there is some uncertainty about the appropriate duration of screening. Screening is also recommended (category 2B evidence) for individuals at least 50 years old with at least a 20 pack-year smoking history plus one additional risk factor; these include: cancer history, lung disease history, radon exposure, and occupational exposure.
The NCCN screening algorithm is based on an adaptation of the Fleishner Society guidelines, NLST data, and the I-ELCAP protocol guidelines. Additional guidelines are included to address subsolid nodules and ground glass opacities which are typically low-grade adenocarcinoma (formerly BAC).
Follow-up of screening protocols are based on the size and morphology of the detected lesion. Solid or part solid lesions are addressed together. Ground glass and nonsolid nodules are addressed separately and are beyond the scope of this article.
Solid or part solid nodules less than or equal to 4 mm in size are followed with yearly LDCT. Nodules 4-6 mm receive follow-up LDCT in 6 months. Nodules 6-8 mm receive follow-up LDCT in 3 months. Nodules greater than 8 mm should have PET/CT. Further work-up is based on findings on follow-up LDCT or PET/CT. Persistent suspicious findings should ultimately be confirmed by tissue diagnosis.
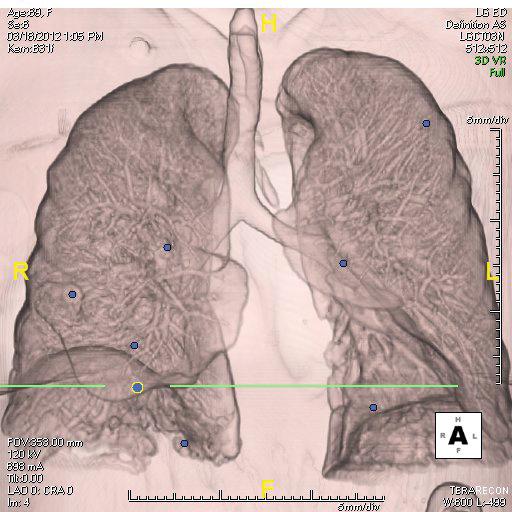
Fig. 1. Computer aided detection (CAD) software identifies multiple pulmonary nodules show in blue.
Benefits of Lung Cancer Screening
Major CT screening studies including NLST and I-ELCAP have reported that 65-85% of their detected lung cancers are stage I. Survival is directly related to stage at the time of diagnosis. Stage IA NSCLC has a 5-year survival of about 75% and drops to less than 25% for stages III and IV.
After 3 years of screening, the NLST showed a 20% reduction in lung cancer mortality compared with screening CXR. The actual reduction in mortality may exceed 20%, since routine CXR is not recommended for lung cancer screening and annual LDCT screening may be continued for more than 3 years.
Risks of Lung Cancer Screening
In NLST 27% of individuals had an abnormal initial screening exam and, as noted earlier, 96% of these were false positive. Though most were assessed with serial LDCT follow-up, some required more invasive yet ultimately unnecessary procedures. And even when an actual tumor is detected, there are still two types of potential concerns. On the one hand is the problem of detection of indolent tumors which may not actually pose a health risk during the patient’s life time. On the other hand, the unique biology of small cell lung cancer means that detection of some aggressive tumors may be futile because they have already metastasized and are incurable by current means.
Cost Effectiveness of Lung Cancer Screening
A recent analysis from the University of Washington assessed national expenditures and cost-effectiveness of lung cancer screening with LDCT based on the NLST data. They found that at current Medicare rates, LDCT screening will add 1.3 to 2.0 billion dollars in annual healthcare expenditures for screening uptake rates of 50 – 75% respectively. LDCT at a 75% screening rate will avoid up to 8100 premature lung cancer deaths per year. The additional cost of screening to avoid one lung cancer death was found to be $240,000. Cost per quality adjusted life year (QALY) is not currently available. McMahon et al found that the cost effectiveness of LDCT screening is markedly improved if the exam is used as a “teachable moment” to successfully encourage smoking cessation. Until cost effectiveness is proven, it is unlikely that Medicare or other insurers will cover the cost of LDCT screening exams.
Lung Cancer Screening at LGH
Based on the recent NCCN guidelines a multispecialty lung cancer screening program has been established at LGH with involvement of physicians from radiology, oncology, pulmonary medicine, thoracic surgery, and radiation oncology. Clinical follow-up and data analysis is coordinated by a dedicated nurse-navigator in much the same way our breast cancer screening program is organized. Weekly case conferences are held to solve problems with difficult cases and to plan interventions if needed.
Most patients will enter the lung cancer screening program through their primary care physician, as self referral is not currently allowed according to regulations of the PA Department of Health. Referring clinicians should counsel their patients on the benefits of screening as well as the possibility of a false positive screen, subsequent management of a positive screen, and the implication of a negative screen. Patients should consider that LDCT screening is not just a test but rather a process that has potential risks associated with the high number of false positive findings. The screening process is best used in conjunction with a program for smoking cessation. Screening LDCT is currently not covered by Medicare or most private insurance carriers, but the total cost is being kept to approximately $300. If the screening exam reveals a positive finding that needs additional follow-up, the subsequent procedures are generally covered by most insurers.
Summary
Lung cancer screening with LDCT is a complex, controversial, and rapidly evolving topic. The NLST showed a 20% decrease in disease-specific mortality with annual LDCTs performed over 3 years. Based on these findings the NCCN recommended screening LDCT in high risk patients. The NCCN guidelines also provide recommendations for evaluation and follow-up of nodules found on screening. The cost effectiveness and benefit-to-risk ratio for lung cancer screening remains to be determined.
Lung cancer screening is best performed in a multidisciplinary program encompassing multiple associated specialties to optimize quality, facilitate decision making, and ensure adequate follow-up. Counseling to assist with smoking cessation should play an important role in this process. At LGH, screening for lung cancer with LDCT is a promising service to the community which hopefully will reduce the mortality of the nation’s leading cause of cancer death.
References
1.The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med 2011; 365:395-409 (Aug. 4)
2.http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
3.The International Early Lung Cancer Action Program Investigators. Survival of Patients with Stage I Lung Cancer Detected on CT Screening. N Engl J Med 2006; 355:1763-1771(October 26)